510(k) Data Packages and Support
iFyber offers both standard and custom preclinical efficacy testing and data packages that can be submitted to FDA in support of substantial equivalence decisions for traditional medical devices and combination products, such as wound dressings and coated devices. iFyber has a GLP-compliant quality system and can provide GLP testing services upon request.
Most performance claims are based on in vitro testing; however, the FDA does not recognize any in vitro antimicrobial effectiveness testing standards that can be directly applied to antimicrobial wound dressings without modification. Companies must define what is appropriate for their specific device and provide adequate justification. All testing must be completed on the final product configuration at the end of the shelf-life and conditioned to emulate factors of clinical use.
iFyber specializes in designing and executing customized test methods suited to your needs. We can help you identify worst-case and clinically relevant test articles, as well as develop and execute customized testing to fit the intended use of your product.
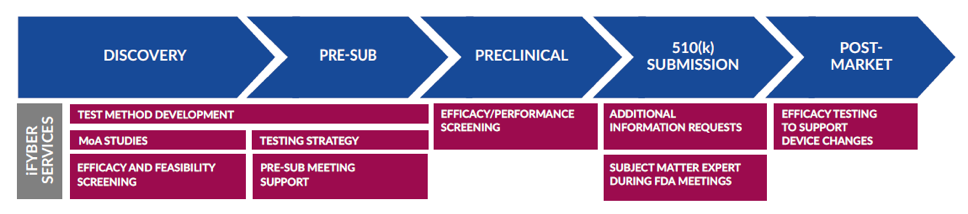
Explore iFyber's 510(k) Data Package Services:
- Efficacy data packages and support for 510(k) Submissions
- Characterization and validation of supercritical CO2 sterilization methods
- In vitro antibacterial efficacy testing
- Performance test method development, selection, and execution
- Modified AATCC test method 100
- Identification of worst-case scenario test articles

