Sterilization Validation Services
To provide safe and sterile products, manufacturers must determine and describe appropriate sterilization cycle parameters through a sterilization validation process. In support of these efforts, iFyber offers customized sterilization validation services for organizations using supercritical carbon dioxide (scCO2) sterilization systems.
During the sterilization validation process, our multidisciplinary team will help guide you in process and cycle optimization, material compatibility studies, measurement of sterilant residuals, and development of microbiological recovery and enumeration methods for validating scCO2 terminal sterilization of your medical device or biological product.
iFyber has a partnership with NovaSterilis to perform validation services on their equipment and is comfortable with other scCO2 technologies and other novel sterilization processes.
Supercritical CO2 Sterilization
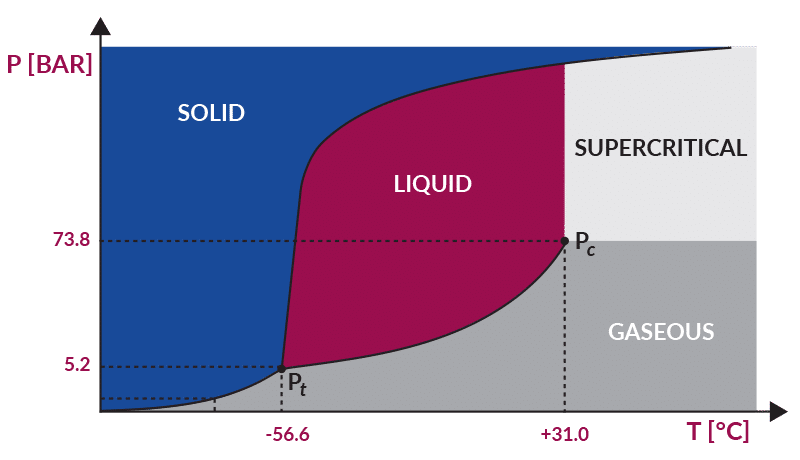
The technology is a novel sterilization method that uses CO2, sterilant additives, and instrumentation to achieve terminal sterilization to a sterility assurance level (SAL) 10-6. When exposed to pressures and temperatures above 73.8 bar and 31°C, respectively, CO2 transitions to a supercritical state, which lends itself to deep penetration of substrates and inactivation of a wide variety of microorganisms.
Through a multi-year collaboration, iFyber has gained an in-depth understanding of the scCO2 sterilization technology and has established a partnership with the NovaSterilis team. We are therefore in the unique position to work directly with both NovaSterilis and our clients to design and execute sterilization validations to ensure that the scCO2 sterilization process will consistently achieve the required sterility assurance level (SAL) for regulatory clearance or approval.
What sterilization validation services can iFyber offer?
All products have unique designs, materials, and packaging configurations. Therefore, the sterilization conditions that may be adequate for one product are likely not sufficient for another. Because of this, all sterilization processes must be validated based on the unique characteristics of the specific product.
iFyber specializes in designing and executing customized sterilization validation test strategies suited to your needs. Our quality, regulatory, and technical teams can help you navigate these requirements through each of the four phases listed below, including characterization of the scCO2 sterilization agent and development and validation of the scCO2sterilization process per ISO 14937, selection of a process challenge device (PCD), and validation of appropriate biological indicators (BI) per ISO 11138.
iFyber has worked closely with NovaSterilis to advance the scCO2 technology through the regulatory approval process. iFyber’s multidisciplinary team, which consists of regulatory professionals, chemists, materials scientists, and biologists, can work with clients to design and execute material characterization, microbiological resistance, and sterilization validation testing plans. To date, iFyber has helped both medical device and biologics customers develop and execute scCO2 sterilization validations to submit for FDA clearance or approval.
Contact us today to discuss your sterilization validation needs.
Sterilization Validation Process
Phase 1: Development
- Material compatibility and characterization of sterilized products
- Characterization testing strategies
- Regulatory support: Q-submissions and representation at FDA Pre-Sub meetings
- Process challenge device (PCD) identification
- Biological indicator (BI) characterization and validation
- Development of microbiological recovery methods for resistance studies
- Draft work instructions and standard operating procedures (SOPs)
Phase 2: Verification
- Develop sterilization process inputs and outputs
- Performance verification
- Quantifying sterilant residuals and chamber mapping
- Survivor curve analysis
- D-value determination
Phase 3: Validation
- Sterilization validation plan
- Installation Qualification/Operational Qualification (IQ/OQ)
- Performance Qualification (PQ)
- Transfer to manufacturing: re-validation or bridging studies
Phase 4: Regulatory Submissions
- Drafting sterilization sections for FDA submissions
- Responses to additional information (AI) requests
- Representation at FDA meeting

Testing Labs
Our BSL-2 testing facilities are fully equipped to handle the sterilization validation testing needs of medical device and biologics companies. We have access to qualified equipment and can work with a variety of challenging microorganisms, including Bacillus atrophaeus and Geobacillus stearothermophilus. We also offer method development services to identify the appropriate microbiological inoculation and recovery methods for your specific product.


