Quality and Regulatory Consulting
Regulatory strategy and quality management system (QMS) implementation are often two of the most challenging aspects of launching a medical device. The process can seem complex and overwhelming for new device developers and, unfortunately, there is no one-size-fits-all guide that defines the exact regulatory path for a specific device. Quality and regulatory consulting provides essential guidance to navigate regulatory requirements and implement effective quality systems.
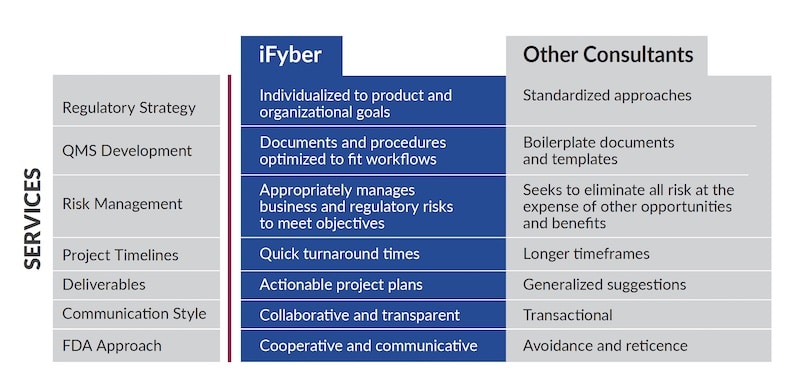
iFyber’s quality and regulatory consultants can help simplify the process to create and implement a manageable, tailored, and cost-effective plan that makes sense for your specific product and business objectives. Whether you need help writing sections of a 510(k) or a comprehensive quality and regulatory strategy for a new device, we can become an extension of your team to provide high-quality deliverables quickly and communicate in a thorough and efficient manner.
Our direct approach to managing the business risks quality and regulatory affairs produce differentiates iFyber from other consultants, and our sponsors and clients value that difference. We believe conversations with regulatory agencies should happen early and often. Communication, paired with a critical examination of the required regulations, standards, and guidances, help our clients efficiently navigate the regulatory landscape with confidence.
Quality and Regulatory Consulting Services
To legally market a medical device in the US, manufacturers must design, develop, test, and manufacture products according to Current Good Manufacturing Practices (cGMPs) and other applicable standards and guidance. In support of these efforts, iFyber offers individualized regulatory and quality consulting services, including strategy development, regulatory submission support, and Quality Management System development.
Regulatory Strategy and Consulting
- Submission strategy development
- Predicate identification for premarket notifications
- 513(g) Requests for Information, device classification
- Intended use and claims
- Identifying applicable regulations and standards
- Informational meetings with FDA
- Quality and Regulatory Consulting Services
Regulatory Submissions and Post-market Activities
- Pre-submission applications
- Investigational Device Exceptions (IDE)
- 510(k) Premarket Notifications
- De Novo Petitions/De Novo 510(k)
- Emergency Use Authorizations (EUA)
- Additional Information requests
- Post-market evaluation of device changes
- Collateral materials and labeling
- Efficacy Data Packages and Support for 510(k) Submissions
- Case Study: Emergency Use Authorization (EUA) of a Decontamination System for Personal Protective Equipment (PPE) During the COVID-19 Public Health Emergency
Quality Management System (QMS) Development and Support
- Quality System Gap Analyses and Audits
- Establishing QSR (21 CFR Part 820)/ISO 13485 compliant quality systems
- Modifying quality system to include FDA/ISO requirements
- Drafting manuals, policies, and procedures
- NCR/CAPA procedures
- Developing work instructions and process flows
- Ensuring appropriate design, change, and document control
- Internal Auditing
- Design and Development Planning
- Design History Files
- Manufacturing documentation and design transfer activities
- Validation master plans, SOPs, and protocols
- Quality Assurance Support and Quality System Training
In addition to regulatory and quality consulting services, iFyber offers both standard and custom preclinical efficacy testing and data packages that can be submitted to FDA in support of a 510(k) submission. iFyber offers both GLP and non-GLP-compliant testing and we specialize in designing and executing customized test methods.
Contact our team today to discuss your quality and regulatory needs.
