Resources
Biofilm Disruptors in Wound Care Products: Moving Beyond Antimicrobials
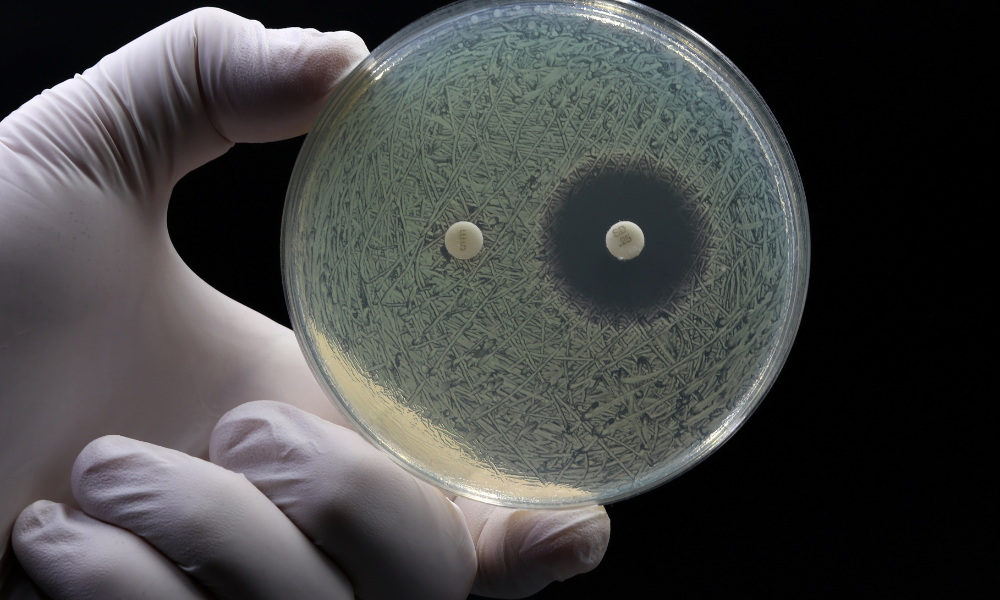
Since the 1940s, antimicrobials have been our first line of defense in wound care. However, the very thing that has helped us fight infections is now starting to help the infections fight back. Structured communities of microorganisms, like bacteria, fungi, or yeast—called biofilms— are significantly more tolerant to antimicrobial treatments and harder to eradicate than free-floating cells. Even though there are effective biofilm disruptors making waves in the wound care field, more popular antimicrobial agents are still predominantly used worldwide. As a result, increased reliance on antimicrobials in biofilm-associated infections is helping drive the rise of antimicrobial resistance.
The solution is to rethink the design of wound care products. To effectively treat chronic wounds and combat antimicrobial resistance, manufacturers are exploring innovative approaches, such as integrating biofilm disruptors, that weaken bacterial defenses and improve treatment outcomes.
Traditional Antimicrobial Strategies in Wound Care
For decades, wound care has relied heavily on antimicrobial agents to control infection and promote healing. Among the most widely used are topical antibiotics, silver-based dressings, and antiseptic washes. These treatments are designed to reduce microbial load, prevent colonization, and create an environment conducive to tissue repair.
However, these traditional strategies show significant limitations in chronic or complex wounds, particularly those complicated by biofilm formation. Antimicrobial agents often demonstrate short-term efficacy; they may kill free-floating bacteria initially but fail to prevent recolonization. Worse, their poor penetration into established biofilms renders them largely ineffective against the microbial communities that shield themselves in these protective matrices. Once a biofilm has formed, even high concentrations of antimicrobials may be insufficient to eliminate the infection.
Compounding the issue is the growing threat of antimicrobial resistance. Frequent or prolonged use of antibiotics and antiseptics increases the likelihood that pathogens will adapt and become resistant to treatment. This not only makes recurring or chronic infections more difficult to treat but also contributes to the global spread of drug-resistant pathogens, which is a serious and escalating public health concern.
A review from the National Library of Medicine points out how these challenges highlight the urgent need to rethink how we approach wound infection. To effectively treat chronic wounds and avoid exacerbating antimicrobial resistance, the next generation of wound care products must do more than kill bacteria. They must disrupt the protective environment that allows them to persist.
Biofilms and Antimicrobial Resistance Without Biofilm Disruptors
Biofilms are structured communities of microorganisms embedded within a self-produced matrix of extracellular polymeric substances, including proteins, polysaccharides, and DNA. This matrix acts as a scaffold that allows bacteria to adhere to wound surfaces and persist in the face of treatment.
Biofilms are associated with the delayed healing and recurrence often observed in chronic wounds. One of their primary defense mechanisms is the physical barrier they create, which limits the ability of antimicrobial agents to reach the bacteria within. This shielding effect can reduce the impact of topical treatments.
Importantly, biofilms are also associated with antimicrobial resistance. The enclosed biofilm environment promotes genetic exchange between bacteria and may increase the likelihood of resistance traits emerging or spreading. As such, biofilms can reduce antimicrobial efficacy while contributing to the broader public health challenge of antimicrobial resistance.
Breaking down the protective biofilm structure can enhance the performance of existing antimicrobials and open the door to non-antibiotic treatment approaches that are less likely to drive resistance.
Engineering Wound Care Products with Biofilm Disruptors
Medical device manufacturers must consider biofilm-resistant strategies during development. This requires a strategic, interdisciplinary design mindset that integrates materials science, microbiology, and surface engineering from the outset.
Choosing the right materials is critical. Factors like surface texture, porosity, and how the material interacts with moisture affect both fluid management and the ability of bacteria to stick to the dressing. Making thoughtful design choices can help create conditions that discourage biofilm formation.
In addition, integrating biofilm disruptors, components designed to weaken the extracellular matrix, into materials or surface coatings can expose the resident bacteria, making them more accessible to treatment. Biofilm disruptors can improve antimicrobial efficacy while reducing reliance on traditional antibiotics.
These combined strategies are crucial for enhancing antimicrobial efficacy and supporting wound healing. iFyber specializes in accelerating wound care innovation through custom, high-quality testing and its services are tailored to answer the most important questions at the earliest stage of development—before costly clinical studies or regulatory submissions.
How iFyber supports product innovation:
- Customized Biofilm Assays
iFyber designs both in vitro and ex vivo models—including high-throughput 96-well biofilm susceptibility assays and ex vivo porcine and human dermal wound models—to simulate clinically relevant environments where biofilms are likely to form. These assays help you understand how your material or formulation performs before it enters complex in vivo systems. - Quantitative Antimicrobial Efficacy Testing
iFyber offers a full range of validated methods—minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) assays, time-kill studies, adhesion and attachment assays, and zone-of-inhibition assays—to quantify how well your product inhibits or eliminates microbial growth, both with and without biofilms present. - Simulated Wound Models
Developing wound care devices requires expertise in microbiology and mammalian cell culture. iFyber’s models go beyond basic planktonic bacterial testing, providing a more comprehensive evaluation of antimicrobial efficacy under wound-relevant conditions. - Surface and Material Characterization
iFyber evaluates the physical and chemical properties of materials—such as hydrophobicity, surface topography, and porosity—that influence microbial adhesion and biofilm formation. This data can inform material selection and support regulatory claims.
Leading developers of wound care products and medical devices know that demonstrating biofilm resistance and antimicrobial efficacy is a critical differentiator. iFyber partners with scientists, engineers, and R&D leaders to help bring these next-generation solutions to life.
Looking for a preclinical testing partner who understands your product challenges? iFyber combines microbiology, surface science, and custom assay development to support medical device teams from concept through commercialization. Reach out today to discuss your goals.

