Resources
Evaluation of Antimicrobial Efficacy
Antimicrobial efficacy testing is crucial for assessing the performance and safety of antimicrobial solutions and products used in clinical settings. These tests evaluate the products’ ability to prevent or inhibit the growth of microorganisms, reducing the risk of healthcare-associated infections (HAIs) and ensuring patient safety. They provide a systematic approach to assess the products’ effectiveness in preventing microbial colonization and biofilm formation. By enhancing infection control practices and improving patient outcomes, these tests promote the safety and effectiveness of healthcare interventions.
Principal Investigator of Microbiology at iFyber, Abdullah Ibn Mafiz, PhD, says, “At iFyber, we provide antimicrobial efficacy testing for a wide range of products, including wound care products, disinfectants, and biomaterials. In addition to standard testing, our greatest strength is that we also offer customized testing for antimicrobial products to address questions that traditional CROs may not be able to answer. Our customers also have direct access to our Ph.D. scientists to discuss and resolve their problems, which is not very common in traditional CROs.” Explore common antimicrobial efficacy testing methods offered by iFyber.
Antimicrobial Efficacy Testing Methods
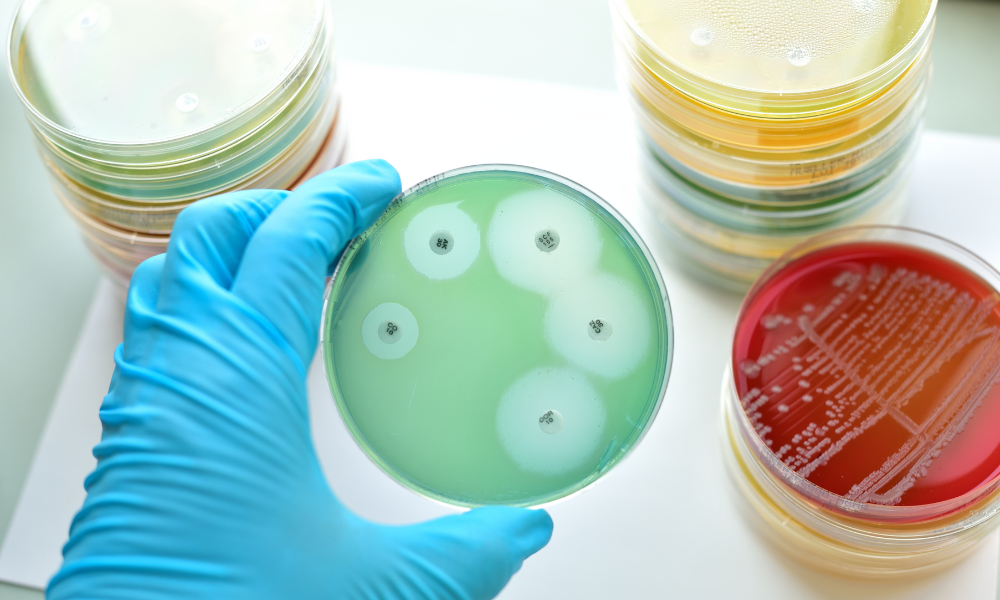
Zone of Inhibition (ZOI)
The Zone of Inhibition (ZOI) test is a fundamental microbiological procedure widely used in the medical device and pharmaceutical industries. It offers a quick and cost-effective way to assess the effectiveness of specific antimicrobial agents against target microorganisms. This method is primarily used to evaluate antimicrobial-releasing designs (leaching). In this test, the samples are placed on agar plates inoculated with target microorganisms. As antimicrobials diffuse into the agar, creating a concentration gradient, microbial growth inhibition zones become visible. The width of these zones indicates the level of antimicrobial activity. Depending on the microorganisms, a 24–48-hour incubation period is typically used to qualitatively evaluate the amount of antimicrobial released and the susceptibility of the microbial strain. The ZOI test can also be used for contact-killing designs by studying microbial growth directly underneath a sample.
MIC/MBC Testing
MIC (Minimum Inhibitory Concentration) and MBC (Minimum Bactericidal Concentration) antimicrobial testing are crucial procedures used to evaluate the effectiveness of antimicrobial agents against microorganisms. MIC determines the lowest concentration of an antimicrobial that inhibits the visible growth of a microorganism, while MBC determines the lowest concentration that kills the microorganism. These assessments are beneficial in the R&D stage of a product for identifying the necessary concentrations needed in the final product. Typically, the concentration of the drug required for efficacy is often significantly lower than that present in the finished dosage form. For MIC/MBC evaluation, different concentrations of the antimicrobials are introduced to cultured microorganisms, and the outcomes are assessed through broth dilution to determine the MIC and MBC values. Normally, MIC/MBC testing involves organisms relevant to product-related infections.
Time-Kill Assay
The Time-Kill assay is a key method in antimicrobial testing, assessing how effectively antimicrobial agents kill microorganisms over time. It involves exposing microorganisms to different concentrations of the antimicrobial agent and sampling over set intervals. The procedure involves exposing the microorganisms to the antimicrobial agent at various concentrations and sampling aliquots at predetermined intervals. These samples are then plated onto agar plates to quantify viable microbial counts. The Time-Kill assay helps establish the antimicrobial agent’s bactericidal or bacteriostatic activity by comparing microbial growth kinetics between treated and untreated samples. This assay is particularly valuable in evaluating the kinetics of antimicrobial action, determining the optimal concentration and exposure time required for effective microbial eradication, and assessing the development of antimicrobial resistance.
Adhesion/Attachment Assay
Adhesion-based antimicrobial assessment evaluates the ability of antimicrobial agents to prevent the attachment and colonization of microorganisms on surfaces. Adhesion-based techniques are well-suited for assessing contact-killing designs. It focuses on inhibiting microbial adhesion, which is crucial in preventing biofilm formation and subsequent infections. This assessment typically involves exposing surfaces to microbial suspensions for a defined period usually 1-4 hours, followed by removing non-adherent bacteria through washing, the collection of adherent bacteria through sonication, and subsequent counting of colony-forming units (CFUs).
Biofilms
Biofilm assessment is vital for antimicrobial evaluation due to the resilience of biofilms against treatments. Biofilms, complex microbial communities encased in a protective matrix, pose challenges in antimicrobial efficacy. Assessing biofilm formation and susceptibility helps in understanding the real-world effectiveness of antimicrobial solutions, guiding the development of strategies to combat biofilm-related infections and improve treatment outcomes. Additionally, biofilm assessment aids in the selection of appropriate antimicrobial agents and formulations that can effectively penetrate and eradicate biofilms, thereby enhancing the efficacy of antimicrobial therapies.
Abdullah mentioned that both in vitro and ex vivo models are available at iFyber to evaluate the prevention and eradication of biofilms by test products. In vitro models utilize high-throughput 96-well plates. For biofilm eradication assessment, 48-hour biofilms are formed in the wells of the plate, followed by exposing the formed biofilms to the test agent for a predefined time. On the other hand, for biofilm prevention, depending on the intended usage of the products, the test agent and biofilm-forming microorganisms are concurrently added to the wells, allowing the microorganisms to grow for a predefined time to determine if the test agent prevents biofilm formation.
The characteristics of biofilms grown on non-biological substrates can differ from those occurring in soft tissue. Therefore, iFyber has adopted an ex vivo porcine dermal model to evaluate both biofilm prevention and eradication. This model is suitable for assessing the efficacy of various products, from antibiotics to medical devices. As porcine skin is physiologically very similar to human skin, this model provides valuable information for screening or optimizing dosing, formulation, and dressing format parameters at a fraction of the cost compared to animal models.
iFyber Offers a Suite of Ready-to-Go Antimicrobial Testing Services
iFyber can go beyond the widely known antimicrobial assessment methods noted above, assisting their customers in solving their product-specific problems and customizing their antimicrobial testing needs. iFyber is a leading contract research organization offering preclinical R&D testing and support in several industries. Explore biofilm and antimicrobial efficacy services or connect with an expert to discuss your questions or needs.

